Communicating with FDA When Data Integrity Issues Arise During Clinical Trials - Food and Drug Law Institute (FDLI)
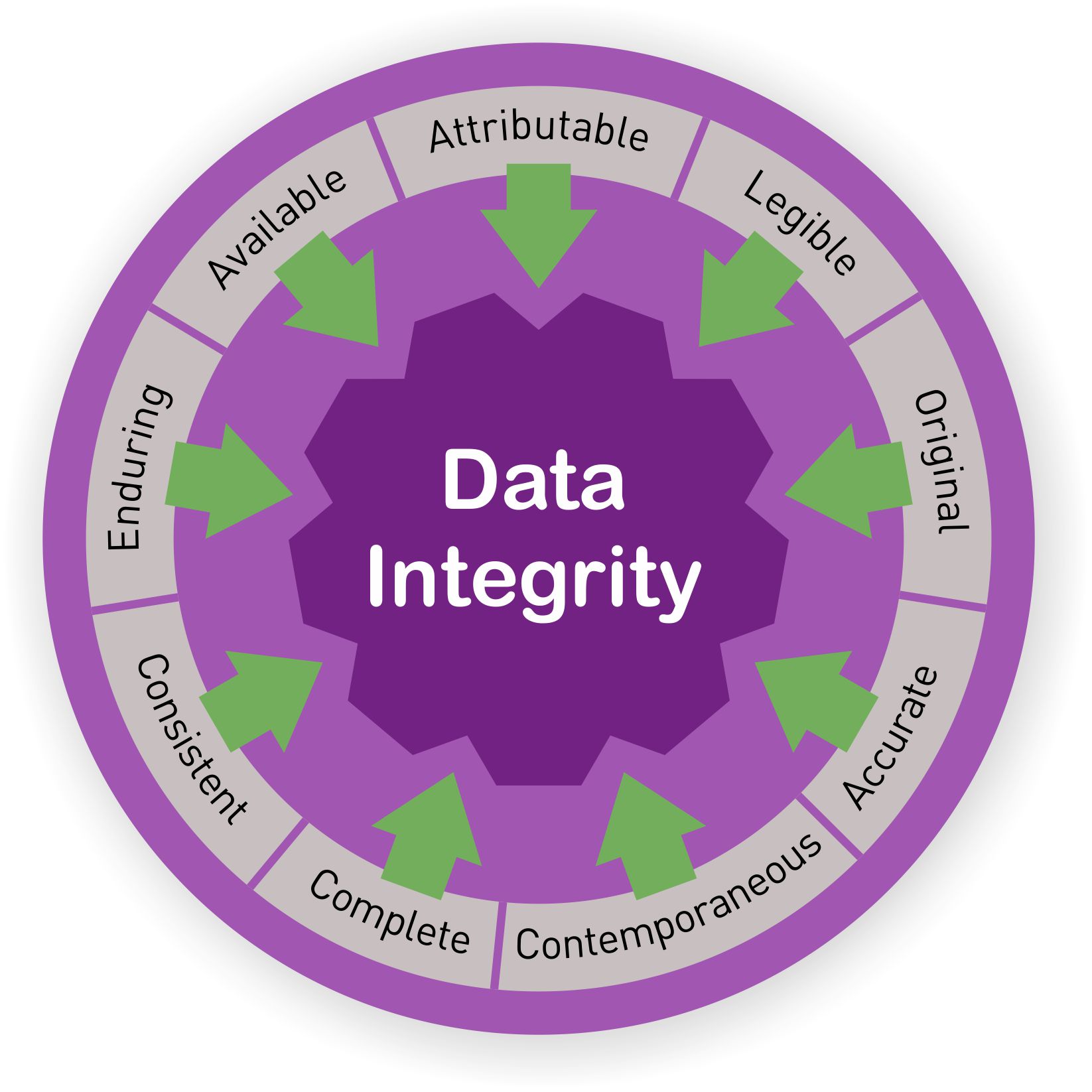
![Data Collected in Clinical Trials is Worthless without Data Integrity [Video] - LearnGxP: Accredited Online Life Science Training Courses Data Collected in Clinical Trials is Worthless without Data Integrity [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2021/03/ELM-903-01-Data-Collected-in-Clinical-Trials-is-Worthless-without-Data-Integrity.png)
Data Collected in Clinical Trials is Worthless without Data Integrity [Video] - LearnGxP: Accredited Online Life Science Training Courses

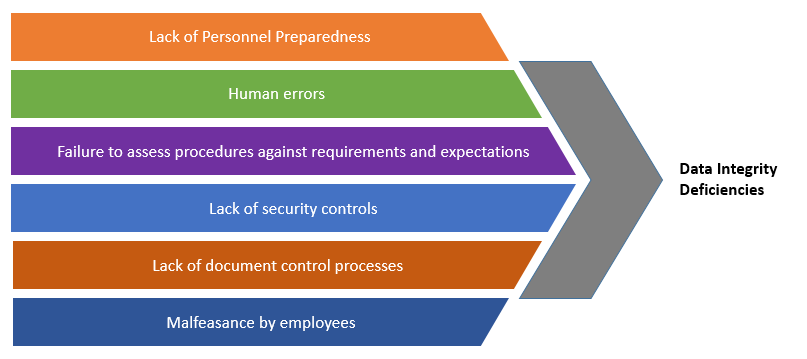
Communicating with FDA When Data Integrity Issues Arise During Clinical Trials - Food and Drug Law Institute (FDLI)

PDF) Data Integrity in Global Clinical Trials: Discussions from Joint US FDA and MHRA UK Good Clinical Practice Workshop